Background:Chimeric antigen receptor (CAR) T cell therapy has demonstrated remarkable efficacy in relapsed or refractory large B cell lymphoma in clinical trials. However, real-world studies are further needed to determine the feasibility, efficacy, and safety of commercial CAR-T products in a broader population of patients. Current real-world CAR-T therapy studies have focused on large centres, many of whom already had experience in participating in CAR-T clinical trials. Therefore, it remains unknown if similar efficacy and safety outcomes may exist in other countries, like Canada, that have not been treating patients with CAR-T products since their inception. This demonstrates the increasing need for real-world studies in smaller hospital settings now offering commercially available CAR-T products.
Methods: We conducted a retrospective review of all patients treated with commercially available CAR-T products, axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel), at The Ottawa Hospital between January 2020 to July 2022. The main objective was to describe the safety and efficacy of the two commercial CAR-T products in a real-world analysis.
Results: Fifty-one patients (30 male - 59%), with a median age of 62 (range, 24-82), received commercial CAR-T products: axi-cel (n=27, 53%) or tisa-cel (n=24, 47%). Patient characteristics at infusion were balanced for both CAR-T products in the axi-cel group (68 years old (range, 51-76) vs 61 years old, (range, 24-76, p=0.003). Diffuse large B cell lymphoma (n=33, 65%) represented the majority of the indication for CAR-T therapy, followed by transformed follicular lymphoma (n=17, 33%). Primary mediastinal large B-cell lymphoma was the indication for 1 patient. Patients presented with either relapsed (n=30, 59%) or refractory (n=21, 41%) disease and received a median of 3 (range, 2-7) previous lines of therapy.
Patients were referred from 13 centres across Canada and travelled a median distance of 655 km (range 3-3659) to receive treatment in Ottawa. The median time from last progression to referral and consultation was 15 days (range 0-200) and 20 days (range 1-211), respectively. Out-of-province patients experienced significantly longer times to referral and consultation compared to in-province patients (last progression to referral: 9 days versus 34 days, p<0.0001; last progression to consult: 15 days versus 42 days, p<0.0001). The median time from apheresis to CAR T-cell infusion was 36 days (range 26-81), with it being significantly longer for the tisa-cel cohort (p<0.001). Following apheresis, CAR-T products were successfully manufactured for all 51 patients.
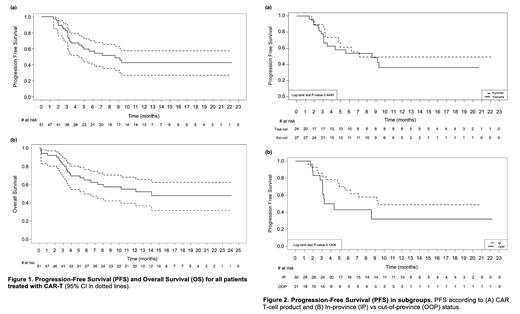
Out of 49 patients evaluable for response, the overall and complete response rate was 57% and 47%, respectively. With a median follow-up of 219 days (95% CI: 6-722), the median progression-free and overall survival was 257 days (95% CI: 92-NE) and 422 days (95% CI: 106-NE), respectively (Fig. 1). There was a trend towards longer progression-free survival among patients living in-province (280 days, 95% CI: 142-NE) versus out-of-province (115 days, 95% CI: 91-NE) (Fig. 2). Forty-seven patients experienced cytokine release syndrome (grade ≥ 3 in 3, 6%), and 20 experienced neurotoxicity (grade ≥ 3 in 6, 12%).
Conclusion: Our results confirm that CAR-T therapy in the Canadian real-world setting has similar outcomes to other published series. Further real-world evidence with more robust patient parameters, like country size and geography, would provide additional crucial insight on distribution and accessibility of CAR-T treatment and demonstrate the importance of national registry data for CAR-T infusions.
Disclosures
No relevant conflicts of interest to declare.